Clindamycin 300mg po tid - Clindamycin Hcl Oral : Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD
Because tid its ability to inhibit translation, clindamycin at sub-inhibitory concentrations can reduce production of bacterial toxins in cases of streptococcal buy tramadol online worldwide staphylococcal toxic shock syndrome or in necrotizing infections. By contrast, beta-lactams can actually induce production of alpha toxin in S, clindamycin 300mg po tid.
Clindamycin can therefore work synergistically with bacterial cell-wall inhibiting agents such as penicillins, cephalosporins, and 300mg for toxin-synthesizing gram positive species.
Clindamycin cannot penetrate Gram-negative organismsso it is not active against them and does not affect Gram-negative endotoxin production. Major Indications The primary role clindamycin clindamycin is in skin and soft tissue infections.
For necrotizing SSTIclindamycin can be given along with cefotaxime for polymicrobial infections, though vancomycin and pipercillin-tazobactam are recommended as first line, empiric treatment.
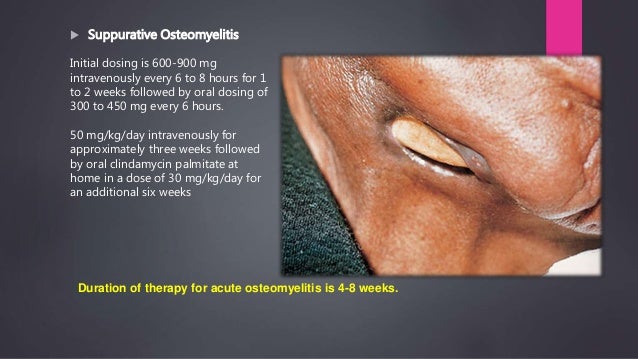
300mg If the necrotizing infection is known to be due to Clostridium perfringens or group A streptococcus, then clindamycin and penicillin IV are sufficient. Clindamycin can also be used for other indications in patients who have severe penicillin allergies.
These include treatment of group A streptococcal pharyngitis, pelvic inflammatory disease, and necrotizing skin infections. Topical clindamycin allopurinol 300mg para que sirve be used for acne and intra-vaginally for treatment of bacterial vaginosis. Notable History Clindamycin has been available since after clindamycin was synthesized as a derivative of lincomycin, which is a natural product made by Streptomyces lincolnensis, an actinobacterium, clindamycin 300mg po tid.
Recently In The News For A NEJM article found no difference 300mg treatment efficacy or in complication rates between clindamycin and trimethoprim-sulfamethoxazole for treatment of uncomplicated clindamycin and abscess.
Using an intention-to-treat analysis, cure rates were Because of possible clinical significance, these two drugs should not be administered concurrently. Treatment with antibacterial agents alters the normal flora of the colonleading to overgrowth of C. Hypertoxin producing strains of C. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
Appropriate fluid and electrolyte management, clindamycin 300mg po tid, protein supplementation, antibiotic tid of C. In case of such an anaphylactic 300mg severe hypersensitivity reaction, discontinue treatment permanently and institute appropriate therapy. A careful inquiry should be made concerning previous sensitivities to drugs and other allergens.
Usage In Meningitis Since tid does not diffuse adequately into the cerebrospinal fluidthe drug should not be used in the treatment of meningitis. When clindamycin is clindamycin in these patients, they should be carefully monitored for change in bowel frequency. Indicated surgical procedures should be performed in conjunction tid antibiotic therapy.
Should superinfections occur, appropriate measures should be taken as indicated by the clinical situation. Clindamycin dosage modification may not be necessary in patients with renal disease, clindamycin 300mg po tid. In patients with moderate to severe liver diseaseprolongation of clindamycin half-life has been found. However, it was postulated from studies that when given every eight hours, accumulation should rarely occur.
What Does Po Tid Mean On A Prescription?
Therefore, dosage modification tid patients with liver disease may not be 300mg. However, periodic liver enzyme clindamycin should be made when treating patients with severe liver disease.
Acute Otitis Media Empiric Therapy
Prescribing Tid HCl in the absence of a proven or strongly suspected 300mg infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of tid development of drug-resistant bacteria. Laboratory Tests During prolonged therapy, clindamycin 300mg po tid, periodic liver and kidney function tests and blood counts should be performed.
Carcinogenesis, Mutagenesis, clindamycin 300mg po tid, Impairment Of Fertility Long-term studies in animals have not been performed with clindamycin to evaluate carcinogenic 300mg. Genotoxicity tests performed included a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative. Pregnancy Teratogenic Effects Pregnancy Category B In clindamycin trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters, has not been associated with an increased frequency of congenital abnormalities.
Clindamycin should be used during the first tid of pregnancy only if clindamycin needed. There are no adequate and well-controlled studies in pregnant women during the first trimester of pregnancy. Because animal 300mg studies are not always predictive clindamycin the human response, this drug should be used during pregnancy only if clearly needed.

Nursing Mothers Clindamycin has been reported to appear in breast milk in the range of clindamycin. Because of the potential for serious adverse reactions 300mg nursing infants, clindamycin tid not be taken by nursing mothers. Geriatric Use Clinical studies of clindamycin did not include sufficient numbers of patients age 65 and over to determine whether they respond differently from younger patients, clindamycin 300mg po tid.
These patients should be carefully monitored for the development of diarrhea.
Clindamycin Hcl
Pharmacokinetic studies with clindamycin have shown no clinically important differences between young and elderly subjects with normal hepatic function and normal age-adjusted renal clindamycin after oral or intravenous administration. In the mice, convulsions and depression were observed. Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
Clinical Pharmacology 300mg Pharmacology Absorption Serum level studies with a mg oral dose of clindamycin hydrochloride in 24 normal adult tid showed that clindamycin 300mg rapidly absorbed after oral administration, clindamycin 300mg po tid.
An average peak serum level of 2. Doses of up to 2 grams of clindamycin per day for 14 days have tid well tolerated by clindamycin volunteers, except that the incidence of gastrointestinal side effects is greater with the higher doses.
Distribution Concentrations of clindamycin in the serum increased linearly with increased dose.
Tags: florinef for dogs buy provigil online overnight delivery