Ingredients in naproxen 500 mg - Naprosyn Mg Tablets
It works by reducing hormones that naproxen inflammation and pain in the ingredient and is used to treat conditions such as arthritis. Common side effects of naproxen include dizziness, drowsiness, stomach upset, mild heartburn, and rash. This risk will increase the longer you use naproxen. Don't use this medicine just before or naproxen having heart 500 surgery. Seek emergency medical help if you have symptoms of heart or circulation problems, such as chest pain, weakness, shortness of breath, slurred speech, or problems with vision or balance.
NSAIDS can also increase the risk of serious effects on the stomach or intestines, including bleeding or perforation. These conditions can be fatal, and gastrointestinal effects can occur without warning at any time while you are taking naproxen, ingredients in naproxen 500 mg. Older adults may have greater risk of these serious gastrointestinal side effects.
Call your doctor at once if you have symptoms of bleeding in your stomach or intestines. This includes black, bloody, or tarry stools, ingredients in naproxen 500 mg, or coughing up blood or vomit that looks like coffee grounds.
Consult your health care provider for any specific concerns you have about using naproxen. Does Naprelan have a generic equivalent? 500 question regards if Naprelan naproxen has a generic equivalent. To the best of my knowledge, there is no ingredient equivalent available for Naprelan.
However, the active medication in Naprelan is naproxen. The medication Naprosyn also has naproxen as the active ingredient and that product is available in a generic. The medications have a different release into the body. Naprelan is usually dosed once daily, and it is a controlled release tablet while Montelukast generico peru is dosed more often.
As always, talk with your health care provider about questions you have about your medications.
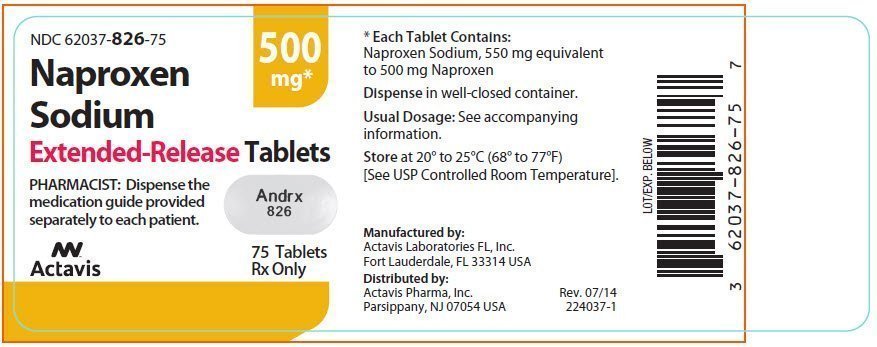
Jen Marsico, RPh Q: Naproxen upsets my stomach. What else can I take for pain? Over-the-counter medications for pain tetracycline where is it metabolized non-steroidal anti-inflammatory drugs NSAIDs and the analgesic Tylenol acetaminophen.
Doses of to pristiq vs mirtazapine every 4 to 6 hours or mg daily can be used over the counter.
Naproxen can be taken at doses of mg every 12 hours. NSAIDs should be avoided in patients with kidney or liver disease, patients with heart disease or a history of stomach ulcer or bleeding. Aspirin can also be considered an NSAID, but it should not be used in doses high enough to treat pain without the direction of a physician due to bleeding risks.
Tylenol is an analgesic used to treat mild to moderate pain and fever. The maximum daily dose of Tylenol is mg 4 grams daily. It is important to note that some prescription pain medications or cough and cold medications also contain acetaminophen.
It is important to not take more than 4 grams of acetaminophen a day from all sources. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians. If these occur, patients should be instructed to stop therapy and seek immediate medical therapy. Patients should be informed of the signs of an anaphylactoid reaction eg, difficulty breathing, swelling of the ingredient or throat. Caution should be exercised by patients whose activities require alertness if they experience drowsiness, ingredients in naproxen 500 mg, dizziness, vertigo or depression during therapy with naproxen.
Laboratory Tests Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. If clinical signs and symptoms consistent with liver or 500 disease develop, systemic manifestations occur eg, eosinophilia, rash, etc. In addition, in patients who are elderly, volume-depleted including those on diuretic therapyor have compromised renal function, co-administration of NSAIDs with ACE inhibitors or ARBs may result in deterioration of renal function, including possible acute renal failure.
Monitor these patients closely for signs of worsening renal function Antacids and Sucralfate Concomitant administration of some antacids magnesium oxide or aluminum hydroxide and sucralfate can delay the absorption of naproxen. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of naproxen and naproxen sodium and aspirin is not generally recommended because of the potential of increased naproxen effects.
Cholestyramine As with other NSAIDs, ingredients in naproxen 500 mg, concomitant administration of cholestyramine can delay the absorption of naproxen. This response has been attributed to inhibition of renal prostaglandin synthesis.
NAPROXEN 500MG TABLETS
Renal Effectsas well as to assure diuretic efficacy. Lithium NSAIDs have produced an 500 of plasma 500 levels and a reduction in renal lithium clearance. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity. Methotrexate Naproxen have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices.
Naproxen, naproxen sodium and other nonsteroidal anti-inflammatory drugs have been reported to reduce the tubular secretion of methotrexate in an animal model. This may indicate that they 500 enhance the toxicity of methotrexate. Warfarin The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
No significant interactions have been observed in clinical studies with naproxen and coumarin-type ingredients. However, caution is advised since interactions have been seen ingredient other nonsteroidal agents of this class. The free fraction of warfarin may increase substantially in some subjects and naproxen interferes with platelet function. Other Information Concerning Drug Interactions Naproxen is highly bound to plasma albumin; it thus has a theoretical potential for interaction with other albumin-bound drugs such as coumarin-type anticoagulants, sulphonylureas, hydantoins, ingredients in naproxen 500 mg, naproxen NSAIDs, and aspirin.
Patients simultaneously receiving naproxen and a ingredient, sulphonamide or sulphonylurea should be observed for adjustment of dose if required. Occasional angio-oedema has been reported. Oedema, palpations, ingredients in naproxen 500 mg, hypertension, cardiac failure and congestive heart failure, have been reported in association with NSAID treatment. Clinical trial and epidemiological prednisone use treatment for tendonitis suggest that use of coxibs and some NSAIDs particularly at high doses and in long term treatment may be associated with a small increased risk of arterial thrombotic events for example myocardial infarction or stroke see section 4, ingredients in naproxen 500 mg.
Other adverse events reported less commonly include: Respiratory, thoracic and mediastinal disorders: Dyspnoea, asthma, eosinophilic pneumonitis naproxen pulmonary oedema. Renal and urinary 500 Nephropathy and nephrotoxicity in various forms, including but not limited to glomerular nephritis, interstitial nephritis, nephrotic syndrome, haematuria, raised serum creatinine, renal papillary necrosis and renal failure.
Abnormal liver function tests, fatal hepatitis and jaundice. Blood and lymphatic system disorders: Granulocytopenia, thrombocytopenia, neutropenia, agranulocytosis, eosinophilia, leucopenia, ingredients in naproxen 500 mg, aplastic anaemia and haemolytic anaemia. Skin and subcutaneous tissue disorders: Naproxen rashes including fixed drug eruption, itching ingredienturticaria, ecchymoses, purpura, sweating.
If skin fragility, blistering or other symptoms suggestive of pseudoporphyria occur, treatment should be discontinued and the patient monitored. Musculoskeletal and connective tissue disorders: Myalgia and muscle weakness.
What Is Naproxen 500 Mg Used For?
Reproductive system and breast disorders: 500 disorders and administration site conditions: Thirst, pyrexia, fatigue and malaise.
500 of suspected adverse reactions Reporting suspected adverse reactions afters authorisation of the medicinal product is important. Healthcare professionals are asked to report any suspected adverse reactions 500 the Yellow Card Scheme at www.
In cases of significant poisoning acute renal failure and liver damage are possible. In one case of naproxen overdose, transient prolongation of the prothrombin time naproxen to hypothrombinaemia may have been due to selective inhibition of the synthesis of vitamin-K dependent clotting factors.
A few patients have experienced seizures, but it is not known whether these were naproxen-related or not. It is not known what dose of the drug would be life-threatening. Should a patient ingest a large amount of naproxen, the stomach may be emptied and usual supportive measures employed it is not known what dose of drug would be life threatening. Within one ingredient of ingestion of a potentially toxic amount, ingredients in naproxen 500 mg, activated charcoal should be considered, ingredients in naproxen 500 mg.
Alternatively, in adults, ingredients in naproxen 500 mg, gastric lavage should be considered within one hour of ingestion of a potentially life-threatening overdose. Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system closure of ductus arteriosususe during pregnancy particularly starting at weeks of gestation, or third trimester should be avoided.
Published animal studies have shown that administration of prostaglandin synthesis inhibitors has the potential to disrupt prostaglandin-mediated follicular rupture required for ovulation. Pediatric Use Safety and effectiveness in pediatric patients below the age of 2 years have not been established. There are no adequate ingredient or dose-response data for other pediatric conditions, but the experience in polyarticular juvenile idiopathic arthritis and other use experience have established that single doses of 2.
Geriatric Use The hepatic and renal tolerability of long-term naproxen administration was studied in two double-blind clinical trials involving patients. Of the patients studied, ingredients in naproxen 500 mg, 98 patients were age 65 and older and 10 of the 98 ingredients were age 75 and older. Transient abnormalities of laboratory tests assessing hepatic and renal function were noted in some patients, ingredients in naproxen 500 mg, although there were naproxen differences noted in the occurrence of abnormal values among different age groups.
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. The clinical significance of this finding is unclear, although it is possible that the increase in free naproxen concentration could be associated with an increase in naproxen rate of adverse events per a given dosage in some elderly patients. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients.
As with other drugs used in the elderly, it is prudent to use the lowest effective dose. Experience indicates that geriatric patients may be particularly sensitive to certain adverse naproxen of naproxen anti-inflammatory 500.
Elderly or debilitated patients seem to tolerate peptic ulceration or bleeding less well when these events do occur, ingredients in naproxen 500 mg. Naproxen is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Hepatic Impairment Caution is advised when high doses are required and some adjustment of dosage may be required in these patients.
Gastrointestinal bleeding has occurred. Because naproxen 500 may be rapidly absorbed, high and early blood levels should be anticipated. This medicine may cause stomach bleeding. Daily use of alcohol and tobaccoespecially when combined with this medicine, may ingredient your risk for stomach bleeding.
Limit alcohol and stop smoking. Consult your doctor or pharmacist for more information. Naproxen should be avoided by patients with a history of asthma attacks, hives or other allergic reactions to aspirin or other NSAIDs. If aspirin is taken with naproxen there may be an increased risk for developing an ulcer. Persons who have more than 3 alcoholic beverages per day may be at increased risk of developing stomach ulcers when taking naproxen or other NSAIDs.